1. 6P_(4)O_(10)+H_(2)O 3H_(3)PO_(4)
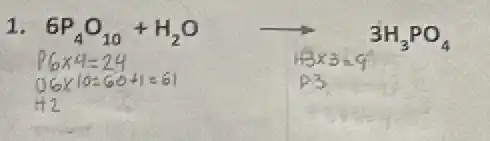
Solution4.5(273 votes)
Answer
Explanation
Similar Questions
How many Sodium ions are in 156 moles of Sodium ions? $9.39\times 10^{23}$ Sodium ions $2.59\times 10^{-24}$ Sodium ions $6.022\times 10^{23}$ Sodium ions 1.56 Sodium ions
Which of the following alcohols will be used in this lab? Butanol Propanol Ethanol Methanol
Acidic dyes work best in low pH environments. are used for staining negatively charged molecular structures. are negatively charged and work best at low pH. are lipid soluble. are negatively charged.
How many protons does carbon have? 40 39.09 6 12
Measurements taken with a spectrometer require a __ to eliminate background readings from components other than what is being measured in the sample. Select one: a. sample b. replicate c. treatment d. blank
11. [Periodic Table and Periodic Law] Which of the following elements has the greatest tendency to attract electrons in a chemical bond? A. Carbon B. Oxygen C. Fluorine D. Nitrogen
Name the following organic compounds: compound name $\square $ $CH_{2}-CH_{2}-CH_{3}\\ \downarrow \\ CH_{3}-CH_{2}-C-CH_{2}-CH_{2}-CH_{3}\\ CH_{3}-CH_{2}-CH_{2}$ $\square $ $CH_{3}-CH_{2}-CH_{2}\\ CH_{3}-CH_{2}-CH_{2}-C-CH_{2}-CH_{2}-CH_{3}\\ \downarrow \\ CH_{2}-CH_{3}$ $\square $ $\square $ $CH_{3}\\ \downarrow \\ CH_{3}-CH_{2}-CH_{2}-CH_{3}\\ CH_{3}-CH_{3}$ $\square $ $\square $
-Develop possible solutions - Brainstorming - think of as many solutions as possible of \( \mathrm{CO}_{2} \) wooden car
8. Every chlorine atom has: 7 electrons 17 neutrons a mass number of 35 an atomic number of 17
What is the maximum number of electrons that can occupy a given orbital if they have different spins? 4 2 1 3









