24. Write a chemical equation that accurately represents what happens when sulfur(S) and oxygen (O_(2)) react to form sulfur trioxide (SO_(3)) Make sure you balance the equation.
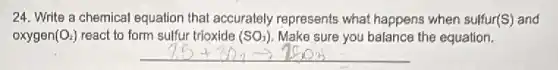
Solution4.4(270 votes)
Answer
Explanation
Similar Questions
True or False: Solutes are things that are dissolved (like salt/sugar) true false
In which type of bond are electrons transferred? covalent ionic
Aluminum produces lots of thick, white smoke. The biggest issue with this smoke is that the fumes associated with aluminum __ sharp Decrease vistathy
What is the mass of 0.513 mol $Al_{2}O_{3}$ Give your answer to the correct number of significant figures. (Molar mass of $Al_{2}O_{3}=102.0g/mol)$ 0.513 mol $Al_{2}O_{3}=\square gAl_{2}O_{3}$
3. How is a reaction described when a chemical reaction uses up energy? endothermic exothermic limiting a product
What is most likely to happen in a single displacement reaction? Two ionic compounds rearrange to form two different compounds. A compound breaks apart into individual elements. Two separate elements combine to form a compound. A metal replaces another metal in a compound.
2. a. What are substances called whose water solutions conduct electricity? b. Why does a salt solution conduct electricity? c. Why does a sugar-water solution not conduct electricity?
2. Name two metalloids commonly used in electronics. 3. Explain why metalloids are important in making semiconductors.
What element in group/family 13 does not have a positive charge? carbon beryllium aluminum boron
6. Chunk 6: Rust Formation Rust forms when iron reacts with oxygen and water, creating a new substance with different properties. Iron only Iron, oxygen, and water Iron and carbon dioxide Iron and heat









