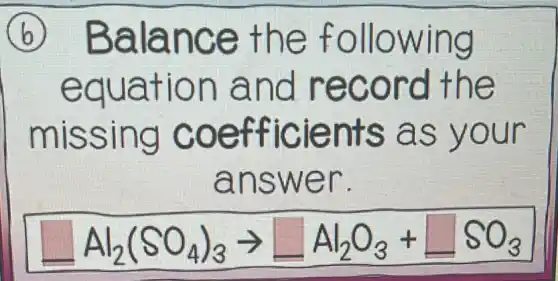
B Balance the following equation and record the missing coefficients as your answer. Al_(2)(SO_(4))_(3)arrow square Al_(2)O_(3)+square SO_(3)
Solution3.7(258 votes)
Answer
Explanation
Similar Questions
Question: If magnesium and powdered aluminum are burning in a lab, what class of fire is this? Choose the correct option and then select Done. Class D Class B Class A Class C
Write a balanced half-reaction for the reduction of aqueous hydrogen peroxide $(H_{2}O_{2})$ to liquid water $(H_{2}O)$ in acidic aqueous solution.Be sure to add physical state symbols where appropriate. $\square $
Station 4 , Precipitation Reactions. Which net ionic equation correctly represents $CaCl_{2}(aq)+Na_{2}CO_{3}(aq)$ $\rightarrow CaCO_{3}(s)+2NaCl(aq)$ $2Na^{+}(aq)+CO_{3}^{2-}(aq)\rightarrow Na_{2}CO_{3}(s)$ $CaCl_{2}(aq)\rightarrow Ca^{2+}(aq)+2Cl^{-}(aq)$ $CO_{3}^{2-}(aq)\rightarrow C(s)+O_{2}(g)$ $Ca^{2+}(aq)+CO_{3}^{2-}(aq)\rightarrow CaCO_{3}(s)$
How are other trends on the periodic table related to non-metal reactivity? a) Non-metal reactivity decreases with ionization energy b) Non-metal reactivity decreases with electronegativity c) Non-metal reactivity increases with ionization energy d) Non-metal reactivity increases with electronegativity
Question 17 of 30 What is the predicted change in the boiling point of water when 2.10 g of barium chloride $(BaCl_{2})$ is dissolved in 5 .50 kg of water? $K_{b}of\quad water=0.51^{\circ }C/mol$ molar mass $BaCl_{2}=208.23g/mol$ i value of $BaCl_{2}=3$ A. $-0.00031^{\circ }C$ B. $0.0028^{\circ }C$ C. $0.00093^{\circ }C$ D. $-0.194^{\circ }C$
What is the mass in grams of 540 moles of lithium? 37.5 6.94 $3.25\times 10^{24}$ 1.29 none of the above
According to the lab guide, which changes below will you look for in order to test the hypothesis? Check all that apply. D changes in shape or size D color changes whether changes are easily reversible temperature changes formation of precipitates or gases changes of state (gas, liquid, or solid)
What is the pH of a solution with a pOH of 7 .0? $pH=[?]$
Is the statement below true or false? The most common form of tetanus is cephalic tetanus. True False
Match the following elements necessary for fire with their corresponding descriptions. CHOOSE AN OPTION BELOW Fuel $\square $ Oxidizing agent $\square $ Heat $\square $ Chemical chain reaction $\square $









