How many moles of CO_(2) are in 2.50times 10^24 molecules of CO_(2)
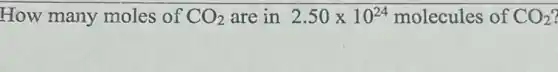
Solution4.3(215 votes)
Answer
Explanation
Similar Questions
One mole of copper has a mass of 63.5 grams. Approximately how many atoms of copper are present in one mole of copper? 63 atoms 64 atoms $32\times 10^{23}atoms$ $6\times 10^{23}atoms$
What is the total pressure in a 10.0 Lflask which contains 0.127 mol of $H_{2}(g)$ and 0.288 mol of $N_{2}(g)$ at $20.0^{\circ }C$ 0.681 atm 0.306 atm 0.998 atm 0.693 atm
What is the final,balanced equation for this reaction? $2Cr^{3+}(aq)+6Cl^{-}(aq)\rightarrow 2Cr(s)+3Cl_{2}(g)$ $2Cr^{3+}(aq)+2Cl^{-}(aq)+6e^{-}\rightarrow Cl_{2}(g)+2Cr(s)$ $Cr^{3+}(aq)+6Cl^{-}(aq)+3e^{-}\rightarrow 2Cr(s)+3Cl_{2}(g)$ $Cr^{3+}(aq)+2Cl^{-}(aq)\rightarrow Cr(s)+Cl_{2}(g)$
Which is an organic thickener? A. Lithium 12 Hydroxystearate B. Silica Gel C. Organo Clay D. Polyurea E. Dithiophosphate F. UNKNOWN
Which of the following functional groups cannot hydrogen bond with itself? alcohol carboxylic acid amine ether
Carbon tetrachloride $(CCl_{4})$ is $\square $ $\square $ ionic.
Given the balanced equations: $Zn+2HCl\rightarrow ZnCl_{2}+H_{2}$ When 1 mole of Zn reacts with 0.8 mol of HCl, which of the following will be true? Zn will be the limiting reactant None of the above Zn will be the excess reactant HCl is the excess reagent
2 mol of an ideal diatomic gas are cooled at constant volume until the pressure is reduced to $1/3$ the initial pressure . What is the entropy change of the gas? A. $-63.9J/K$ B $+63.9J/K$ C. $-9.13J/K$ D. 0 E. $-45.6J/k$
1. Identify the elements located in each position on the periodic table. a. period 3, group 16 b. period 5, group 9 c. period 6, group 18
The number of moles present in 180 grams of hydrochloric acid is approximately __ 4.75 5.00 4.25 4.50









